- Research
- Open access
- Published:
Operationalizing and digitizing person-centered daily functioning: a case for functionomics
BMC Medical Informatics and Decision Making volume 24, Article number: 184 (2024)
Abstract
An ever-increasing amount of data on a person’s daily functioning is being collected, which holds information to revolutionize person-centered healthcare. However, the full potential of data on daily functioning cannot yet be exploited as it is mostly stored in an unstructured and inaccessible manner. The integration of these data, and thereby expedited knowledge discovery, is possible by the introduction of functionomics as a complementary ‘omics’ initiative, embracing the advances in data science. Functionomics is the study of high-throughput data on a person’s daily functioning, that can be operationalized with the International Classification of Functioning, Disability and Health (ICF).
A prerequisite for making functionomics operational are the FAIR (Findable, Accessible, Interoperable, and Reusable) principles. This paper illustrates a step by step application of the FAIR principles for making functionomics data machine readable and accessible, under strictly certified conditions, in a practical example. Establishing more FAIR functionomics data repositories, analyzed using a federated data infrastructure, enables new knowledge generation to improve health and person-centered healthcare. Together, as one allied health and healthcare research community, we need to consider to take up the here proposed methods.
Introduction
Omics research and the definition of functionomics
An ever-increasing amount of health, healthcare and related research data on a person’s daily functioning is collected by a variety of stakeholders: people themselves, healthcare professionals and researchers, among others. By joint analysis of these data a tremendous amount of information can be derived from these data and has the potential to revolutionize person-centered prevention and healthcare, potentially improving health and life expectancy. Within the fields of oncology, radiology and genetics, computerized analysis of high-throughput data has already shown benefits for the personalization and optimization of healthcare [1,2,3]. These initiatives are often referred to as ‘omics’ research, where analyses of big data on genes (genomics), RNA (transcriptomics), proteins (proteomics), metabolites (metabolomics), and imaging (radiomics) are performed to advance personalized and preventive health and healthcare [4, 5]. To make this possible, ‘omics’ initiatives rely more and more on machine actionable data.
In this study we propose the integration of a new ‘omics’, namely functionomics. The current ‘omics’ research field focusses on biomedical or internal exposures, whilst functionomics can specifically contribute to eliciting interactions with personal and general external exposures (Table 1).
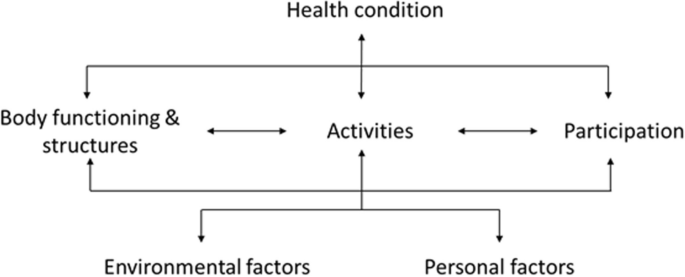
The concept of functioning and its underlying phenomena are globally described by the World Health Organization (WHO) in the International Classification of Functioning, Disability and Health (ICF) [9] (Fig. 1).
The ICF is used internationally in different types of interdisciplinary healthcare and social research settings, as well as to inform health policy development [12, 13]. Therefore the ICF is an ideal framework for providing a common format for making functionomics data machine actionable in an international setting.
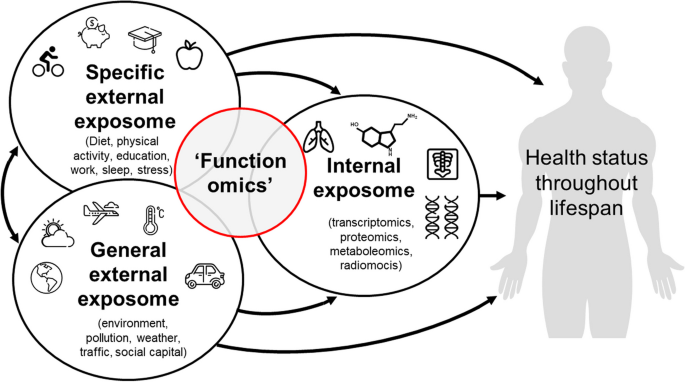
Until now the potential of functionomics data cannot yet be fully exploited, as they are usually stored in an unstructured manner in all sorts of mostly inaccessible data-silos. The lack of machine actionable data makes it difficult for people themselves as well as for outsiders (those not involved in the data collection and storage) to access, understand, analyze, interpret, and reuse these data. Imagine your own hard drive which holds all sorts of research datasets which cannot be accessed or understood by others. This prohibits joint analysis of data, causing dilution of information and loss of valuable knowledge which may result in suboptimal clinical decisions and ultimately less effective care [14]. Therefore, a transition towards the integration of functionomics as an additional ‘omics’ initiative, and at the same time embracing the advances in data science and information technology (IT), is necessary. Integrating functionomics in health, healthcare, education and research practice has an additional benefit on top of the other ‘omics’, as it provides a means to capture a more holistic view of health, rather than the limited biomedical view. Functionomics research can specifically contribute to eliciting interactions with personal and general external exposures (Fig. 2) and to broaden the scope of person-centered healthcare.
The human exposome reflects the totality of internal and external exposures within a human life cycle. Current ‘omics’ research field focus solely on biomedical or internal exposures, whilst functionomics can specifically contribute to eliciting interactions with personal and general external exposures. (adapted from Vrijheid et al. [15])
Functionomics in the context of allied health professionals
Allied healthcare disciplines are well-positioned to pioneer a functionomics initiative, as these disciplines generate large amounts of data that can be captured within the ICF. Allied healthcare comprises a large group of health professionals, that are not physicians, with the core focus of enabling people to enjoy optimal functioning in their daily lives (e.g., physiotherapists, dieticians, speech therapists). For example: there are currently annually 3.84 million people treated by approximately 35 000 physiotherapists in the Netherlands and there are 560 000 physiotherapists in the European Union (EU) [16, 17] and 216 920 in the United States (US) [18]. If we assume that the average physiotherapist generates approximately 0.1 GB of data per patient, [19] we can estimate a data volume of roughly 375 terabytes per year in the Netherlands, 5.9 petabytes in the EU and 2.4 petabytes in the US. Translating these data into information that is actionable at the point of care and subsequently using that information to guide prognosis, diagnosis, prevention, and treatment pave the way towards more adequate and personalized physiotherapy [20, 21]. However, this huge amount of functionomics data can only be processed by machines. Subsequently, acceleration of knowledge generation can only be achieved by making data machine actionable.
Barriers to implementation
To enable functionomics research, there are four major challenges in data collection, processing and storage that need to be addressed: 1) variability in data collection and storage strategies, 2) lack of implementation of community data standards, 3) ethical and social dilemmas like patient privacy issues, and 4) interoperability between IT systems [22]. In this paper we will focus on suggesting solutions for these challenges, where we will focus on the problem that functionomics data are currently not machine actionable as they are collected in a mostly unstructured manner and stored in inaccessible data-silos. The potential to compromise patient privacy when linking records across data silos is an additional complicating factor (challenge 3). These issues could be resolved by creating a federated functionomics data infrastructure before functionomics research can live up to its full potential and will be discussed in this paper.
Transition from data storage to data use
A robust data infrastructure between the many data silos is a prerequisite for any ‘omics’ initiative, as it allows joint analysis of multiple data sources. Such a data infrastructure relies on usage of a ontology and data processing. Particularly, data should be transformed following the FAIR (Findable, Accessible, Interoperable, and Reusable) principles [23]. FAIR principles are internationally promoted as best practice in data management, with examples of successful application in other types of ‘omics’ initiatives [24]. FAIR principles are recommended by organizations like WHO, G20, European Commission, and European Open Science Cloud [25]. Computational ontologies and Semantic Web technologies, are strongly recommended methods to help achieve FAIR data [26]. Applying these methods will provide citizens, health professionals and researchers with machine readable data that can be analyzed via a federated data infrastructure. These concepts are currently under-utilized, as many are unfamiliar with them and what they can bring to daily life challenges up to clinical practice quests.Moreover, many of the prerequisites for making functionomics data FAIR are currently not available in this field. Combined efforts are needed to resolve these issues.
Therefore, the aim of this article is to provide a step-by-step guide on how to implement and utilize FAIR functionomics data, by proposing a method for creating an ontology based on the ICF and introducing internationally advocated concepts (FAIR principles operationalized through Semantic Web technology) for making data machine actionable. In the discussion we will address remaining issues for making data FAIR within the domain of functionomics.
Materials and methods
In this study we used a single database example from a retrospective cohort study, to walk through the steps of creating FAIR data ready for federated analysis. The data were collected with the goal of developing a decision-support system to aid in the personalization of the perioperative care pathway by identifying which patients are at risk for worse short- and long-term outcomes [27]. This study was assessed by the local medical ethical committee AzM/UM (METC AzM/UM) and was considered not applicable to the Medical Research Involving Human Subject Act (number 2019 − 1426). In Table 2, we provide the reader with a glossary of some fundamental terms and abbreviations used in this paper.
A practical example
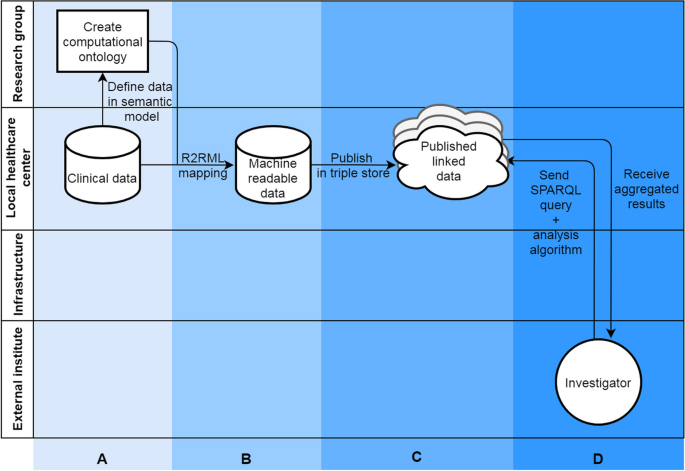
We will describe the first steps in the methodological process to develop a FAIR functionomics database, using the above mentioned dataset, by: A) creating a computational ontology using the ICF, B) making data machine readable, C) publishing data on the Semantic Web to transform clinical data into FAIR and linked data, and D) analyzing data (queried) using a federated learning infrastructure (Fig. 3). The letters A till D in Fig. 3 are used throughout the Methods and Results sections to delimit the different steps in the process.
Case description
Routine clinical data from 160 adult patients were collected during the perioperative care period for patients with degenerative disorders of the lumbar spine opting for fusion surgery. The database contained a set of diverse variables: patient demographic characteristics, patient-reported pain and functioning (including activities), and other clinical outcome measures (Table 3).
Ontology
An ontology was formulated (column A, Fig. 3) to make the data from case study interoperable. The created ontology only provides classes for concepts in our used case. It should be viewed as an example of how the allied health research community can approach building a functionomics ontology. In an ontology, a research field agrees on formal definitions of the terms in the domain and relations among them and are expressed in machine readable language [28]. A machine readable language means that computers can easily find, ‘read’ and understand data, without manual intervention. In our study, we used the open access Protégé (Stanford University, Stanford, CA, USA) software, which incorporates current standards for developing machine readable ontologies: Resource Description Framework Schema (RDFS) and the Web Ontology Language (OWL). Herein we combined terms from existing terminologies in the biomedical field to give universally agreed-upon definitions and structure to our dataset: SNOMED-CT, and Units of Measurement Ontology (UO). The ICF was used as an upper level class structure for our ontology. We added classes from SNOMED-CT and UO to define specific concepts that were available in these ontologies for variables in our dataset (e.g., age, sex). For biopsychosocial variables that could not be defined using the existing ontologies, we formulated a new class. The basic idea of this mapping process was to link each data structure (row, columns and values) within the database to its corresponding component (concept, property, relationship). The way variables are interlinked was defined within the ontology and was based on clinical expertise and understanding of these relationships by the authors. These components were developed using feedback loops with experts in the field of lumbar spinal fusion (LSF), perioperative care and the ICF. The reader should keep in mind that this is only an example, ontologies are flexible and can easily incorporate new variables and relationships or adjust existing variables/relationships. Ideally an ontology should be based on international community standards and consensus.
Semantic web technologies
Semantic Web technologies are an extension of the World Wide Web (WWW) and provide people with a means of publishing and storing data on the Web. Within the Semantic Web, data are described in triples, based on the Resource Description Framework (RDF; column B, Fig. 3). A triple consists of three components, namely: a subject, a predicate and an object. Each of these components has a semantic definition, defined within the ontology. These three components from the defined ontology are combined to make a triple, for example see Table 4:
In a relational database, all variables within a two-dimensional table (e.g., csv file, excel file, SPSS file) have a relation to each other, which needs to be defined in the process of making data machine readable. In this study we used R2RML descriptions to transform our data into RDF triples using the Ontop software package. Once in the dataset all data were transformed into RDF triples, the triples were stored on a web platform called GraphDB (Ontotext, Sofia, Bulgaria) running on the hospitals’ intranet (column C, Fig. 3). We checked the triple mapping using the visual graph interface of GraphDB. The intranet is a private part of the WWW, accessible only to employees of the hospital. The GraphDB instance held the RDF triples and hosts a REST API to receive requests to query the data hosted in the GraphDB instance. The universal language that can be used to query data transformed into RDF triples is SPARQL.
The Personal Health Train (PHT) [10] federated infrastructure allows a researcher or other external parties to perform analyses on data from multiple GraphDB instances or data silos without physically having access to the data (column D, Fig. 3). Through the REST API in the PHT infrastructure a researcher can send their analysis to one or more data stations communicating with a central PHT server. Subsequently the analysis is performed locally in data stations (e.g. hospitals, physiotherapy practices) and only aggregated results are sent back to the researcher via the same infrastructure. This infrastructure can be utilized to send all different types of data analyses – queries and algorithms – to the data stations, like quality assessment, prediction modelling or effectiveness calculations. A SPARQL query and algorithm for performing a simple count of gender was written and performed via the federated infrastructure.
To assess the FAIRness of the data (e.g., the degree to which the digital resource adheres to the FAIR data principles) the data was analyzed using the FAIRMetrics [11]. We used the standardized FAIR maturity indicators manual assessment, which assesses Findability, Accessibility, Interoperability and Reusability of the resource using thirtyfour indicators.
Results
As this paper aimed to provide guidance on how to implement functionomics in clinical practice, a step-by-step tutorial of the described results was created in our GitHub repository: https://github.com/ERCJanssen/Functionomics. Readers can use this tutorial including dummy data similar to the real dataset to recreate the same steps themselves.
Ontology
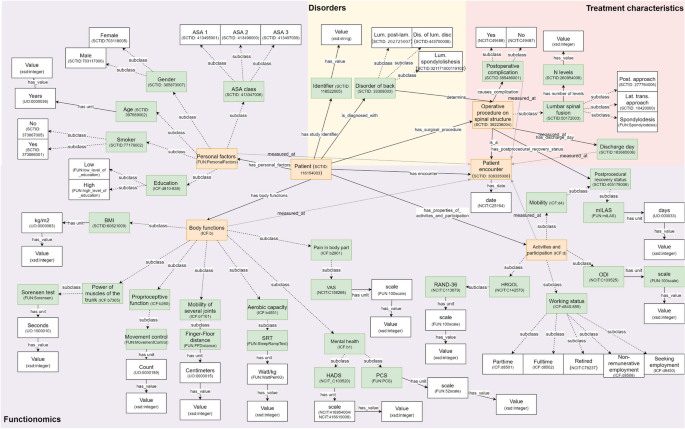
We developed an ontology describing basic concepts, relationships and properties within the preoperative context of a patient deciding forLSF (column A, Fig. 3).The ICF was used as the upper level hierarchy of classes for this ontology, containing 1,596 classes. We added ‘new’ lower level concepts to the ICF structure when these concepts, defining the variables in our dataset, were not available in the ICF. We mapped all variables from our dataset as concepts in the ontology reusing concepts from well-known published ontologies, wherever possible (e.g., SNOMED CT and UO).If no appropriate concepts or relationships were available in existing ontologies, which was often the case for data about a patient’s daily functioning, new concepts were added. In total we added 42 classes and 10 predicates to the ontology. From this process we can see that many of these ‘new’ concepts about a patient’s daily functioning can appropriately be mapped to the ICF hierarchy. This ontology was published on Github (Fig. 4). The ontology can be (re)used and fine-tuned by others to fit their data on a person’s daily functioning.
Basic concepts and relationships within the example dataset, defined within different existing ontologies. Abbreviations: ASA American society of anesthesiology, BMI Body mass index, DIS Disease, HADS Hospital anxiety and depression scale, HRQOL Health related quality of life, KG Kilograms, LAM Laminectomy, LAT Lateral, LUM Lumbar, mILAS Modified iowa level of assistance scale, ODI Oswestry disability index, PCS Pain catastrophizing scale, POST Posterior, SRT Steep ramp test, TRANS Transversal, VAS Visual analogue scale
Using semantic web technology
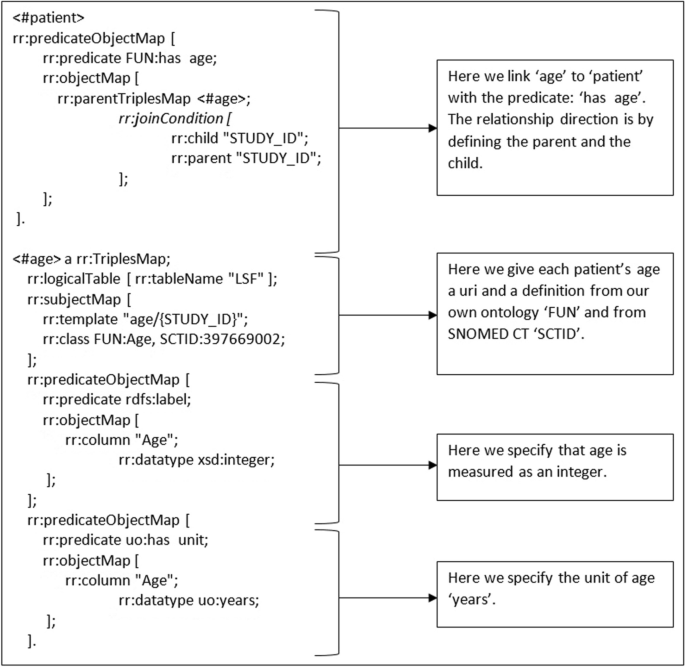
To transform the .csv dataset into machine readable data (RDF triples) we made an R2RML script (column B, Fig. 3). This script reads the .csv file, using the previously created ontology, and is translated it into 74 triples. An example of the mapping file is shown in Fig. 5. The full mapping can be found on GitHub.
The RDF mapping and data were published in a GraphDB instance on a local server, which linked the data repository to the web (column C, Fig. 3). From this point on, data could be analyzed by external parties by linking to the GraphDB instance via the PHT infrastructure (column D, Fig. 3).The PHT infrastructure allows the researcher to perform analysis without having to physically collect the data in a central server [29]. To perform such an action we linked two computers via internet in a password secured infrastructure to prevent unauthorized access to the data. A researcher then sent the example SPARQL query and algorithm to our GraphDB instance via the PHT infrastructure from their own computer. The results of this query were calculated locally in our local GraphDB instance. Subsequently, the aggregated results – frequencies of gender - of this simple query were sent back the researcher via the PHT infrastructure: N females = 101, N males = 59.
FAIRness of the data is described in GitHub repository. The main focus of this example was on the interoperability part of the FAIR principles, as such the scoring for FAIRness metrics on policies is low.From the applicable indicators we scored 7/8 for findability, 2/3 for accessibility, 7/7 for interoperability and 1/4 for reusability.
Discussion
Recent history shows the usefulness of big data analysis in personalizing healthcare through ‘omics’ research in many medical fields [5, 30, 31]. In our practical example we redefined functionomics to include data on daily functioning of a person and showed how it can be operationalized and used, here in a clinical setting. A functionomics ontology for the specific setting and population of the example was created, based on the ICF. Both biomedical and psychosocial data were transformed into a machine readable language (RDF) and published on the web (Graph DB instance). Next, these data were queried (using SPARQL) and gender counts were generated via an analytic algorithm. This paper and the tutorial in the accompanying GitHub repository enables others to familiarize themselves with the proposed approach, establish their own functionomics data station and send all different types of analyses to these stations. Using this approach we will be able to create a network of linked FAIR functionomics datasets.
From its conception many scientific breakthroughs have been established through ‘omics’ research. For example, in the recent years, radiomics has made a serious impact on personalization of radiotherapy, due to firm investment in available IT and statistical solutions [2]. This has resulted in multiple scientific and clinical advancements; for example, an internationally validated prediction model for cancer survival has been developed and new knowledge on tumor phenotypes has been generated [31, 32]. However, when considering the whole human exposome, major concepts are often not included in these ‘omics’ research types: the specific and general external exposome, and a considerable amount of the biopsychosocial aspect of the internal exposome. To further improve health and healthcare research, all elements of the human exposome should be included, informed by a biopsychosocial perspective.
In our practical example, we suggested how to operationalize this transition towards functionomics by using the ICF for the development of an appropriate ontology. The ICF is an international framework and terminology often used by allied healthcare professions to describe and organize data on a patient’s daily functioning. However, the transition from the ICF to a functionomics ontology requires to solve some major gaps in knowledge established in our study. Firstly, a classification of personal factors is lacking in the current ICF class hierarchy and – although different articles are published with preliminary lists - the WHO has decided to refrain from a classification of personal factors in the near future [9]. Secondly, no predicates were available in the ICF to establish relationships between classes. Thirdly, some concepts are hard to map within the current ICF class hierarchy, as they involve multiple ICF classes. In the community there is disagreement on methods of measuring functioning and how to map different concepts to the ICF [33]. Mapping the perception of one’s quality of life, for example, has led to some discussion about its position in the class hierarchy in our practical example as well as in other research, [34] even when applying the linking rules of the ICF [35]. Without consensus on this issue, it will remain difficult to make functionomics data FAIR. Therefore, we propose to address these gaps in knowledge in an international and interdisciplinary collaboration, to enable structured capture of real-world functionomics data. By addressing these issues, we can make functionomics operational, firstly in datasets and field examples, and step by step around the globe.
Making data FAIR has scientific value with a tremendous impact on population health, healthcare and the economy. The cost of not making data FAIR comes at a high price; annually around €100 billion is lost due to missed innovation opportunities [36]. We invest large amounts of time and effort in data capturing, but these data are only operable for single-use purposes, as they are mostly captured in an – when considering a global scale – unstructured and inaccessible manner. The FAIR principles, operationalized in Semantic Web Technology, guide the development of a global infrastructure and tooling to make all health and research data optimally reusable for machines and people alike resulting in the internet of FAIR data and services, where data, far more divergent than just health and research, can be found, accessed, and (re)used by anyone [25]. Accomplishing this will revolutionize the scientific and societal value of this data.
A major advantage of applying Semantic Web technologies and building a functionomics ontology is the ability to link different silos of data and concurrently to query them. In our example an external researcher was able to query our data without it leaving the data silo based in the hospital. Moreover, the researcher only received the aggregated results and not the individual patient data, meaning it is privacy preserving. Applying these techniques can help to solve the issues of physical data integration.
Ultimately, this approach could lead to ‘digital twins’, where one would be in the possession of very detailed biopsychosocial information of a person over time and relate them to similar persons who already underwent diagnostic, prophylactic and/or therapeutic interventions for their health challenges and very accurately predict their health outcomes [37].
Possible barriers for implementation of functionomics
An important issue that we have not addressed in this paper is unstructured, free text, data describing a person’s functioning. Often data on functioning are not collected in a structured manner, as from our example. Concepts of functioning, including the influencing contextual factors (personal and environmental factors), are hard to capture in a cohesive whole using measurement tools. There are two ways we can deal with this issue. The first one is investing in making functionomics data more structured, for example by creating new validated measurement tools and implementing these tools in standard clinical and research practice. However, as mentioned above, data on functioning is very context sensitive, using measurement tools we may lose this context and may not accurately present the patient’s perspective [38]. The second approach is to apply free text mining, like natural language processing (NLP), to extract meaningful concepts from the free text and convert them to structured formats.
Our current science landscape does not promote data and knowledge sharing [39]. This issue is inherent to putting great value on impact factors, publication numbers and grant acquisition. A major worry for many is that when data are shared too early, others will foreshadow their work [40]. Another issue is the analysis of privacy sensitive healthcare data, stored at many different locations. Functionomics data are often collected on the same person by different healthcare providers, social organizations or even by people themselves. Combining these privacy sensitive data repositories for functionomics research requires a privacy-preserving approach. By using federated learning techniques we could largely solve this issue, as it enables local analysis of data with only aggregated results leaving the place of storage, through privacy-by-design. Still, it is obligatory to gain informed consent of any individual to use their healthcare data for research purposes. This would not be feasible in the proposed system, as different types of queries could be sent to the data silo on a daily basis. A tiered informed consent may be a viable solution. Here people grant permission for the (research) purposes of their choice, but not for all [41].
Another thing to keep in mind is that FAIR is not equal to Open: The ‘A’ in FAIR stands for ‘Accessible under well-defined conditions’ [42]. Even when publishing data on the Semantic Web it is still stored locally on a ‘private’ network. The ‘owner’ of the data can still control who gets access to them, in our case through the PHT network, for example by requiring password authentication and authorization. In contrast, opening up data (Open Access) yields most benefits, as it provides researchers access to large amounts of data to analyze.
The next steps in functionomics
Big data analysis is not only a way to improve the robustness of science today, but can drive new scientific discovery of tomorrow. The analysis of big data on functionomics will give valuable insight in how to move forward in personalizing healthcare. For this, an internationally accepted functionomics ontology should be built, capturing all relevant data from the ICF, open access mapping scripts, a trustworthy data infrastructure and international agreements on data usage policies. Therefore, we call to action to all stakeholders in functionomics to contribute to a new ontology and participate in making their (own) data more FAIR.
Conclusion
In this study functionomics as the study of high-throughput data on daily functioning, as defined and objectified in the ICF, is introduced. Functionomics research can have great benefits for health and person-centered healthcare, thus improving health of people and with people. Investments, by an international community in the domain of functionomics, in the proposed IT solutions for big data analysis - FAIR principles through Semantic Web technologies - are necessary to achieve this. Together, as one united health and care (research) community, we need to make serious efforts to take up the proposed methods.
Availability of data and materials
The data that support the findings of this study are not openly available due to reasons of sensitivity. Data are, however, available from the authors upon reasonable request and with permission from the local medical ethical committee (METC UM/AzM). Data are located in controlled access data storage at Maastricht UMC+.
References
Cusumano D, Dinapoli N, Boldrini L, Chiloiro G, Gatta R, Masciocchi C, et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol Med. 2018;123(4):286–95.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Reviews Clin Oncol. 2017;14(12):749–62.
Rhrissorrakrai K, Koyama T, Parida L. Watson for genomics: moving personalized medicine forward. Trends Cancer. 2016;2(8):392–5.
Ibrahim R, Pasic M, Yousef GM. Omics for personalized medicine: defining the current we swim in. Expert Rev Mol Diagn. 2016;16(7):719–22.
Micheel CM, Nass SJ, Omenn GS, editors. Evolution of translational omics: lessons learned and the path forward. Washington (DC): National Academies Press (US); 2012.
Vailati-Riboni M, Palombo V, Loor JJ. What are omics sciences? In: Ametaj BN, editor. Periparturient diseases of dairy cows: a systems biology approach. Cham: Springer International Publishing; 2017. p. 1–7.
Research N, Transcriptomics. Nature Research; https://www.nature.com/subjects/transcriptomics.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–77.
Heerkens YF, de Weerd M, Huber M, de Brouwer CPM, van der Veen S, Perenboom RJM, et al. Reconsideration of the scheme of the international classification of functioning, disability and health: incentives from the Netherlands for a global debate. Disabil Rehabil. 2017;40(5):603–11.
Beyan O, Choudhury A, Soest Jv, Kohlbacher O, Zimmermann L, Stenzhorn H, et al. Distributed analytics on sensitive medical data: the personal health train. Data Intell. 2020;2(1–2):96–107.
Wilkinson MD, Sansone S-A, Schultes E, Doorn P, Bonino da Silva Santos LO, Dumontier M. A design framework and exemplar metrics for FAIRness. Sci Data. 2018;5(1):180118.
Stucki G. International Classification of Functioning, disability, and health (ICF): a promising framework and classification for rehabilitation medicine. Am J Phys Med Rehabil. 2005;84(10):733–40.
Kostanjsek N. Use of The International Classification of Functioning, Disability and Health (ICF) as a conceptual framework and common language for disability statistics and health information systems. BMC Public Health. 2011;11 Suppl 4(Suppl 4):S3. https://doi.org/10.1186/1471-2458-11-S4-S3.
Krumholz HM. Big data and new knowledge in medicine: the thinking, training, and tools needed for a learning health system. Health Aff (Millwood). 2014;33(7):1163–70.
Vrijheid M. The exposome: a new paradigm to study the impact of environment on health. Thorax. 2014;69(9):876–8.
(KNGF) KNGvF. FysioFacts. https://www.kngf.nl/KNGF/Missie+%26+Visie/feiten--cijfers.html; 2019.
Eurostat. Healthcare personnel statistics - dentists, pharmacists and physiotherapists https://ec.europa.eu/eurostat/statistics-explained/index.php/Healthcare_personnel_statistics_-_dentists,_pharmacists_and_physiotherapists2020.
Elflein J, Number of physical therapists in the U.S. 2001–2016 https://www.statista.com/statistics/185731/number-of-physical-therapists-in-the-us-since-2001/#:~:text=Number%20of%20physical%20therapists%20in%20the%20U.S.%202001%2D2016&text=In%202001%2C%20there%20were%20126%2C450,were%20216%2C920%20physical%20therapists%20employed.2016
Lustberg T, van Soest J, Jochems A, Deist T, van Wijk Y, Walsh S, et al. Big data in radiation therapy: challenges and opportunities. Br J Radiol. 2017;90(1069):20160689.
Lambin P, Zindler J, Vanneste BGL, De Voorde LV, Eekers D, Compter I, et al. Decision support systems for personalized and participative radiation oncology. Adv Drug Deliv Rev. 2017;109:131–53.
Bates DW, Saria S, Ohno-Machado L, Shah A, Escobar G. Big data in health care: using analytics to identify and manage high-risk and high-cost patients. Health Aff. 2014;33(7):1123–31.
Skripcak T, Belka C, Bosch W, Brink C, Brunner T, Budach V, et al. Creating a data exchange strategy for radiotherapy research: towards federated databases and anonymised public datasets. Radiother Oncol. 2014;113(3):303–9.
Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR Guiding principles for scientific data management and stewardship. Sci Data. 2016;3(1):160018.
Berrios DC, Beheshti A, Costes SV. FAIRness and usability for open-access omics data systems. AMIA Annu Symp Proc. 2018;2018:232–41.
Mons B, Neylon C, Velterop J, Dumontier M, da Silva Santos LOB, Wilkinson MD. Cloudy, increasingly FAIR; revisiting the FAIR data guiding principles for the European open Science cloud. Inform Serv Use. 2017;37:49–56.
Traverso A, van Soest J, Wee L, Dekker A. The radiation oncology ontology (ROO): publishing linked data in radiation oncology using semantic web and ontology techniques. Med Phys. 2018;45(10):e854–62.
Janssen ER, Osong B, van Soest J, Dekker A, van Meeteren NL, Willems PC, Punt IM. Exploring Associations of Preoperative Physical Performance With Postoperative Outcomes After Lumbar Spinal Fusion: A Machine Learning Approach. Arch Phys Med Rehabil. 2021;102(7):1324–1330.e3. https://doi.org/10.1016/j.apmr.2021.02.013.
Gruber TR. A translation approach to portable ontology specifications. Knowl Acquisition. 1993;5(2):199–220.
Choudhury A, Janssen E, Bongers BC, van Meeteren NLU, Dekker A, van Soest J. Colorectal cancer health and care quality indicators in a federated setting using the personal health train. BMC Med Inf Decis Mak. 2024;24(1):121.
Jochems A, Deist TM, van Soest J, Eble M, Bulens P, Coucke P, et al. Distributed learning: developing a predictive model based on data from multiple hospitals without data leaving the hospital – a real life proof of concept. Radiother Oncol. 2016;121(3):459–67.
Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5(1):4006.
Jochems A, Deist TM, El Naqa I, Kessler M, Mayo C, Reeves J, et al. Developing and validating a survival prediction model for NSCLC patients through distributed learning across 3 countries. Int J Radiat Oncol*Biol*Phys. 2017;99(2):344–52.
Alford VM, Ewen S, Webb GR, McGinley J, Brookes A, Remedios LJ. The use of the international classification of functioning, disability and health to understand the health and functioning experiences of people with chronic conditions from the person perspective: a systematic review. Disabil Rehabil. 2015;37(8):655–66.
Cieza A, Stucki G. Content comparison of health-related quality of life (HRQOL) instruments based on the international classification of functioning, disability and health (ICF). Qual Life Res. 2005;14(5):1225–37.
Stucki G. ICF linking rules: an update based on lessons learned. J Rehabil Med. 2005;37(4):212–8.
Mons B. Invest 5% of research funds in ensuring data are reusable. Nature. 2020;578(7796):491.
Bruynseels K, Santoni de Sio F, van den Hoven J. Digital Twins in Health Care: Ethical Implications of an Emerging Engineering Paradigm. Front Genet. 2018;9:31.
Newman-Griffis DR, Hurwitz MB, McKernan GP, Houtrow AJ, Dicianno BE. A roadmap to reduce information inequities in disability with digital health and natural language processing. PLOS Digit Health. 2022;1(11):e0000135.
Tenopir C, Dalton ED, Allard S, Frame M, Pjesivac I, Birch B, et al. Changes in data sharing and data reuse practices and perceptions among scientists worldwide. PLoS ONE. 2015;10(8):e0134826.
Popkin G. Data sharing and how it can benefit your scientific career. Nature. 2019;569(7756):445–7.
Eisenhauer ER, Tait AR, Rieh SY, Arslanian-Engoren CM. Participants’ understanding of informed consent for biobanking: a systematic review. Clin Nurs Res. 2019;28(1):30–51.
Landi A, Thompson M, Giannuzzi V, Bonifazi F, Labastida I, da Silva Santos LOB, Roos M. The A of FAIR–as open as possible, as closed as necessary. Data Intell. 2020;2(1–2):47–55.
Acknowledgements
We would like to thank dr. A.J. Kittelson for proof reading the paper and providing us with valuable feedback.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
EJ, IP, JvS and NvM contributed to the conception of functionomics and design of the work. EJ and JvS performed data acquisition and analysis. IP, JvS, YH, HS, HtN, LvR, PW and NvM helped interpret the data according to the ICF ontology. JvS, AD, BM and NvM helped interpret the data in the context of FAIR data and semantic web technology. EJ drafted the initial manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki. This study was assessed by the local medical ethical committee AzM/UM (METC AzM/UM) and was considered not applicable to the Medical Research Involving Human Subject Act (number 2019 − 1426). Participant informed consent was obtained under the declaration of no objection.
Consent for publication
n/a.
Competing interests
Johan van Soest reports a relationship with Medical Data Works B.V. that includes: equity or stocks. Andre Dekker reports a relationship with Medical Data Works B.V. that includes: equity or stocks. The other authors have no competing interests to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Janssen, E.R., Punt, I.M., van Soest, J. et al. Operationalizing and digitizing person-centered daily functioning: a case for functionomics. BMC Med Inform Decis Mak 24, 184 (2024). https://doi.org/10.1186/s12911-024-02584-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-024-02584-2